In the realm of chemical synthesis, particularly within organic chemistry and the production of psychoactive substances, PMK oil synthesis from PMK (Para-Methoxyphenylacetone)—CAS number 28578-16-7—has garnered significant attention. This process is complex, requiring precise control over reaction conditions, expert knowledge of chemical pathways, and a thorough understanding of safety protocols.
This comprehensive guide aims to elucidate the intricate process of synthesizing PMK oil from PMK CAS 28578-16-7, explore the chemical mechanisms involved, discuss safety considerations, and provide insights into practical applications. Whether you are a chemist, researcher, or enthusiast, this article will serve as a valuable resource.
Table of Contents
- Understanding PMK and Its Significance
- Chemical Properties of PMK CAS 28578-16-7
- Overview of PMK Oil and Its Uses
- Legal and Safety Considerations
- The Synthesis Pathway of PMK Oil from PMK
- 5.1. Raw Material Preparation
- 5.2. Oxidation Process
- 5.3. Purification Techniques
- Step-by-Step Synthesis Procedure
- Analytical Methods for Quality Control
- Applications and Market Demand
- Environmental Impact and Waste Management
- Conclusion
- References and Further Reading
1. Understanding PMK and Its Significance
PMK (Para-Methoxyphenylacetone) is a key precursor in the synthesis of various psychoactive substances, notably MDMA (Ecstasy) and MDA. Its chemical structure features a phenyl ring with a para-methoxy group attached to an acetone backbone, making it a versatile intermediate in organic synthesis.
The significance of PMK extends beyond illicit drug synthesis; it also plays a role in pharmaceutical research, organic chemistry education, and chemical manufacturing processes. Due to its pivotal role, understanding how to synthesize PMK oil from PMK CAS 28578-16-7 is crucial for chemists involved in controlled and safe chemical production.
2. Chemical Properties of PMK CAS 28578-16-7
Chemical Name: Para-Methoxyphenylacetone
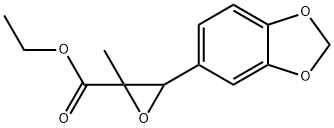
CAS Number: 28578-16-7
Molecular Formula: C_10H_12O_2
Molecular Weight: 168.20 g/mol
Physical State: Typically appears as a clear to pale yellow liquid
Boiling Point: Approximately 220°C (at atmospheric pressure)
Solubility: Slightly soluble in water; soluble in organic solvents like ethanol, acetone, and ether
Chemical Structure:
O
||
Ph–CH2–CO–CH3
|
OCH3 (para position)The para-methoxy group enhances the compound's reactivity and influence on subsequent chemical reactions, making it suitable for further transformation into targeted compounds. Phenylacetone
3. Overview of PMK Oil and Its Uses
PMK oil refers to the crude or refined form of PMK used as a chemical intermediate. It can be extracted or synthesized in bulk, serving as a building block for further chemical reactions.
Key Uses:
- Chemical Intermediary: For synthesizing psychoactive substances, pharmaceuticals, or research chemicals.
- Research Applications: Studying reaction mechanisms involving phenylacetone derivatives.
- Industrial Manufacturing: Production of specialty chemicals.
Note: It is imperative to emphasize that the synthesis and use of PMK are subject to legal restrictions in many jurisdictions. This guide focuses solely on the chemical and scientific aspects.
4. Legal and Safety Considerations
Legal Status:
PMK (Para-Methoxyphenylacetone) and its derivatives are controlled substances in numerous countries due to their potential use in illicit drug manufacturing. Always verify local regulations before handling or synthesizing these chemicals.
Safety Precautions:
- Use personal protective equipment (PPE) including gloves, goggles, and lab coats.
- Conduct reactions in a well-ventilated fume hood.
- Handle chemicals with care to prevent inhalation, ingestion, or skin contact.
- Properly dispose of waste according to local environmental regulations.
5. The Synthesis Pathway of PMK Oil from PMK
The synthesis of PMK oil from PMK CAS 28578-16-7 involves multiple steps, primarily oxidation, which transforms the precursor into the desired product.
5.1 Raw Material Preparation
- Starting Material: High-purity PMK (CAS 28578-16-7)
- Reagents: Oxidizing agents such as potassium permanganate (KMnO₄), sodium dichromate (Na₂Cr₂O₇), or alternative oxidants.
- Solvents: Acetone, ethanol, or other suitable organic solvents.
5.2 Oxidation Process
The core transformation involves oxidizing the methyl group attached to the phenyl ring or the acetone backbone, depending on the targeted intermediate or final product.
Common oxidative pathways include:
- Oxidation of the Side Chain: To generate phenylacetic acid derivatives, which are then converted into PMK oil.
- Oxidation of the Ketone: To control the oxidation level and produce specific compounds suitable for subsequent reactions.
5.3 Purification Techniques
Post-reaction, the mixture contains by-products, unreacted reagents, and impurities that need removal. Techniques include:
- Liquid-liquid extraction
- Recrystallization
- Distillation
- Chromatography
6. Step-by-Step Synthesis Procedure
- Put 47,5 liters of H2O in the reactor and add 2,5kg of sodium bicarbonate, stirring until complete dissolution, take out the mixture and keep it.
- Put in the reactor 7L of H2O
- Add while stirring 1,2kg of sodium hydroxide
- While the H2O and the sodium hydroxide are mixing and getting temp put 5kG of wax PMK to melt by low heat.
- When PMK is melted add it to the reactor and put the mixture at 80 °C reflux during 1 hour stirring.
- Add 3,25kg of HCL 37% in small portions, once all added keep the mixture at 80 °C reflux during 1 hour stirring.
- Stop stirring and put the mixture at 25 °C.
- Discard the water top layer.
- Put again in the reactor the oil top layer.
- Add the H2O and sodium bicarbonate mixture, stirring 2-3 minutes.
- Add 10 liters of DCM (from freezer preferably).
- Let it get separated around 10-15 min.
- Evaporate DCM from the bottom layer
PMK oil (MDP2P; cas 4676-39-5) yield is 3,72kg
Note: This section provides a generalized overview; actual laboratory procedures require detailed protocols, safety data sheets, and regulatory compliance. Bmk oil
Step 1: Dissolution of PMK
Dissolve a known quantity of PMK in an appropriate solvent, such as acetone or ethanol, under stirring.
Step 2: Addition of Oxidant
Slowly add the oxidizing agent (e.g., KMnO₄) while maintaining temperature control (around 0-25°C). Vigorous stirring ensures uniform oxidation.
Step 3: Monitoring the Reaction
Track the reaction progress via Thin Layer Chromatography (TLC) or other analytical techniques like GC-MS.
Step 4: Quenching the Reaction
Once complete, quench excess oxidant with reducing agents (e.g., sodium bisulfite) to prevent over-oxidation.
Step 5: Extraction and Purification
Extract the product using organic solvents, wash, dry over anhydrous agents (e.g., sodium sulfate), and purify via distillation or chromatography.
Step 6: Final Product Collection
Collect the purified PMK oil, verify purity with analytical methods, and store under appropriate conditions.
7. Analytical Methods for Quality Control
To ensure the integrity and purity of synthesized PMK oil, several analytical techniques are employed:
- Gas Chromatography-Mass Spectrometry (GC-MS): For purity and structural confirmation.
- Nuclear Magnetic Resonance (NMR) Spectroscopy: To confirm chemical structure.
- Infrared (IR) Spectroscopy: For functional group verification.
- High-Performance Liquid Chromatography (HPLC): For quantification.
8. Applications and Market Demand
PMK oil serves as a crucial intermediate in various chemical industries:
- Pharmaceutical Research: As a precursor in drug development.
- Chemical Manufacturing: Production of specialty chemicals.
- Research Chemicals: In academic and industrial laboratories.
While some applications are legitimate, the compound's association with illicit drug synthesis has led to stringent regulations and declining legal availability in many regions.
9. Environmental Impact and Waste Management
Chemical processes involving oxidation can produce hazardous waste, including heavy metal residues and organic solvents. Proper disposal and waste treatment are essential to minimize environmental impact.
- Use environmentally friendly oxidants when possible.
- Implement waste segregation protocols.
- Comply with local environmental laws.
10. Conclusion
The synthesis of PMK oil from PMK CAS 28578-16-7 is a sophisticated chemical process that demands a thorough understanding of organic synthesis techniques, safety protocols, and legal considerations. While the process holds significance in research and industrial applications, it must be approached responsibly, adhering to all applicable laws and safety standards.
This guide has provided an in-depth exploration of the chemical pathways, techniques, and considerations involved in PMK oil synthesis. For practitioners, continuous learning, rigorous safety practices, and ethical conduct are paramount.
11. References and Further Reading
- Organic Chemistry Textbooks: For foundational reaction mechanisms.
- Chemical Safety Data Sheets (SDS): For handling reagents.
- Regulatory Agencies: Such as DEA, EPA, for legal guidelines.
- Research Articles: On oxidation techniques and phenylacetone derivatives.
- BMK Oil and Related Resources: bmk-oil.com for commercial information.
Disclaimer:
This article is for informational purposes only. The synthesis and handling of PMK and related compounds are illegal in many jurisdictions without proper authorization. Always consult legal experts and comply with local laws.

Comments
Post a Comment